- FOR US HEALTHCARE
PROFESSIONALS only - Prescribing
Information - Patient
Site
AZSTARYS® is available for $60 or less for all commercially insured patients—see savings offer for eligible patients.

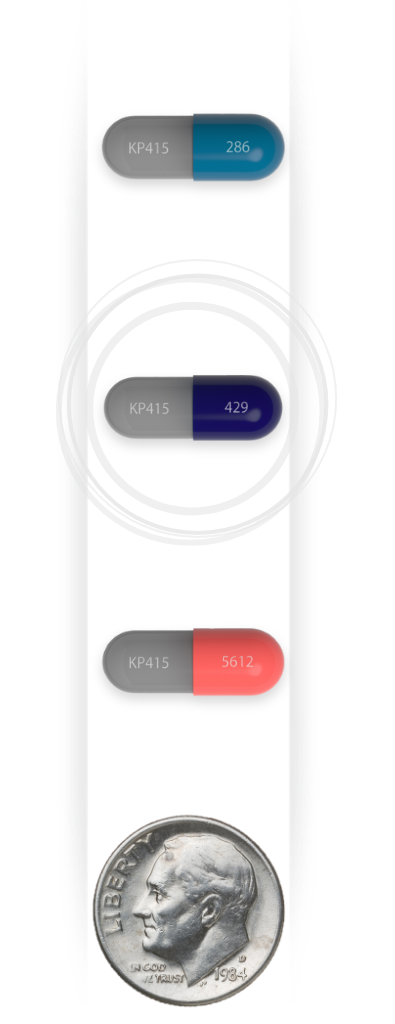
Recommended starting dose once daily
39.2 mg/SDX
IR d-MPH
| AZSTARYS (SDX/ d-MPH) |
Combined molar dose over the day (d-MPH HCl) |
|---|---|
| 26.1 mg/5.2 mg | 20 mg |
| 39.2 mg/7.8 mg | 30 mg |
| 52.3 mg/10.4 mg | 40 mg |

AE=adverse event; d-MPH=dexmethylphenidate; HCl=hydrochloride; IR=immediate-release; MPH=methylphenidate; PK=pharmacokinetic; SDX=serdexmethylphenidate.
Reference: 1. AZSTARYS. Prescribing information. Corium LLC; 2025.
AZSTARYS is a central nervous system (CNS) stimulant indicated for the treatment of Attention Deficit Hyperactivity Disorder (ADHD) in patients 6 years and older.
The use of AZSTARYS is not recommended in pediatric patients younger than 6 years of age because they had higher plasma exposure and a higher incidence of adverse reactions (e.g., weight loss) than patients 6 years and older at the same dosage.
WARNING: ABUSE, MISUSE, AND ADDICTION
AZSTARYS has a high potential for abuse and misuse, which can lead to the development of a substance use disorder, including addiction. Misuse and abuse of CNS stimulants, including AZSTARYS, can result in overdose and death and this risk is increased with higher doses or unapproved methods of administration, such as snorting or injection.
Before prescribing AZSTARYS, assess each patient’s risk for abuse, misuse, and addiction. Educate patients and their families about these risks, proper storage of the drug, and proper disposal of any unused drug. Throughout treatment, reassess each patient’s risk and frequently monitor for signs and symptoms of abuse, misuse, and addiction.
Please click here for Full
Prescribing Information,
including Boxed WARNING on Abuse, Misuse, and Addiction.

AZSTARYS is a central nervous system (CNS) stimulant indicated for the treatment of Attention Deficit Hyperactivity Disorder (ADHD) in patients 6 years and older.
The use of AZSTARYS is not recommended in pediatric patients younger than 6 years of age because they had higher plasma exposure and a higher incidence of adverse reactions (e.g., weight loss) than patients 6 years and older at the same dosage.
WARNING: ABUSE, MISUSE, AND ADDICTION
AZSTARYS has a high potential for abuse and misuse, which can lead to the development of a substance use disorder, including addiction. Misuse and abuse of CNS stimulants, including AZSTARYS, can result in overdose and death and this risk is increased with higher doses or unapproved methods of administration, such as snorting or injection.
Before prescribing AZSTARYS, assess each patient’s risk for abuse, misuse, and addiction. Educate patients and their families about these risks, proper storage of the drug, and proper disposal of any unused drug. Throughout treatment, reassess each patient’s risk and frequently monitor for signs and symptoms of abuse, misuse, and addiction.
The site you're about to enter is intended for US healthcare professionals only.
By selecting OK to continue, you confirm you are a healthcare professional.